Acidity
Wine & Fruit Acids
“Fruit acid” is not a scientific term, but it is a term that has practical use. Most fruit acids are alpha-hydroxy-acids (AHAs), dicarbonic acids, or they can be both. It is important to understand that not all fruit acids are equal when it comes to taste. In fact, fruit acids can have very different mouth-feels.
In winemaking, the most important fruit acids are, in alphabetical sequence:
- citric acid,
- fumaric acid,
- gluconic acid,
- glycolic acid
- α-hydroxycaprylic acid,
- lactic acid, malic acid,
- mandelic acid,
- oxalic acid,
- salicylic acid,
- and tartaric acid
There are other acids that can develop during the fermentation process, but these acids are undesirable (for example, acetic acid).
These acids only develop when fermentation goes astray.
Fermentation can go astray if micro-organisms other than yeast have become involved in the fermentation process or if the appearance of oxygen has led ethanol to oxidize into acetic acid. If the oxidized wine has a high enough alcohol content, the acetic acid can react with the ethanol to produce ethyl acetate, which will give a nail polish flavour to the wine. This flavour is obviously not what we want.
To find out more about other fruit acids during winemaking, please click here.
Wine Acidity
As the diagram below shows, the acidity of fruits can vary.
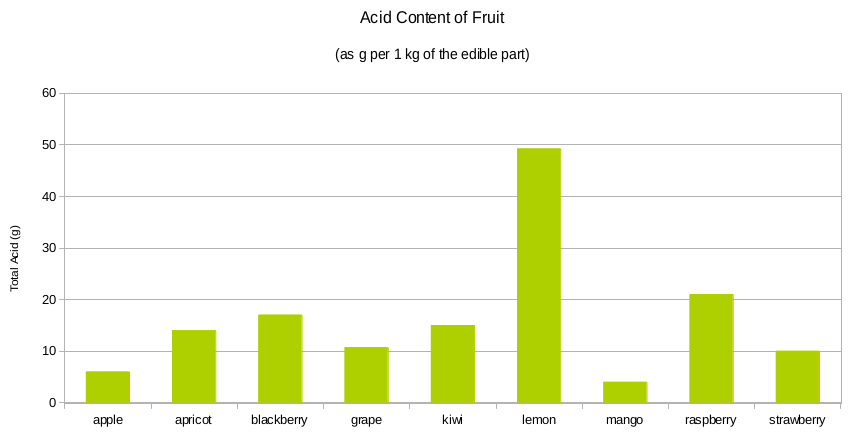
The acidity of wine gives wine its body and balance. For this reason, wine acidity must be controlled properly – just right before start of fermentation, with little room for errors.
Unfortunately, there is no simple way to measure acidity (it is normally done by titration). However, it is easy to estimate the amount of acid that has to be added in winemaking; this is described in the recipes provided in the Recipes section. All fruit species have “acid signatures,” they operate based on the types and concentrations of acids, with little variation in them.
Wine acidity can be measured as “total titratable acidity” (TTA). Since tartaric acid is the most significant acid in winemaking, TTA is commonly expressed as tartaric acid equivalents (TAE).
The Osorno lab can perform TTA analyses. The minimum sample volume required for TTA analyses is 100 ml. Please contact us for more information about wine analyses.
Table 1: TAE Conversion Factors of Fruit Acids
| Acid | Conversion Factor |
|---|---|
| Acetic | 0.20 |
| Citric | 1.92 |
| Fumaric | 0.77 |
| Lactic | 0.39 |
| Malic | 0.89 |
| Oxalic | 0.60 |
| Tartaric | 1.00 |
As you can see in Table 1, 1 g of citric acid corresponds to about double the amount of tartaric acid.
With the TTA of wine, you can distinguish between volatile acids (e.g: acetic acid) and non-volatile acids (e.g: tartaric acid). Obviously, non-volatile acids are by far dominant in wine.
Wine Acidity and pH
Despite common belief, pH and acidity do not have a linear relationship, (because the pH scale is logarithmic), so statements like “10 grams of citric acid will increase the wine’s pH by 0.2” are nonsense. One can certainly calculate the pH value of wine, however, the question will still be what the benefit might be. After all, pH is less significant than the wine’s total acidity and what chemists call the buffer capacity of the wine. Without delving too deep into chemistry, let’s just say that the buffer capacity of wine is related to the wine’s mineral content, which in turn is determined by the wine’s fruit to water ratio.
The buffer capacity of wine also influences the mouth-feel and body of the wine. If necessary, you can increase the buffer capacity of wine by adding certain minerals. Some wines such as mead and dandelion wine are notoriously mineral deficient. This deficiency cannot be compensated by simply adding mineral-rich water to the wine, because these waters will most likely still be lacking a sufficient concentration of potassium. Potassium hydrogen tartrate (also known as “cream of tartar”) will serve this purpose.
The pH value of wine is largely influenced by the wine’s fruit acids; some fruit acids are “more acidic” than others. The “more acidic” is chemically expressed by what is called the pKa value (acid dissociation constant); the table below shows pKa for some select acids.
The lower the value in the table, the stronger the acid. For dibasic and tribasic acids, the given value refers to the first stage only.
Wine Acidity Correction
If wine acidity needs correction, one can add either tartaric acid, lactic acid, or citric acid. These acids are listed in the sequence of preference in which they should be used. Unfortunately for one’s wallet, this is the reverse sequence of price, with tartaric acid being the most expensive fruit acid, and citric acid the cheapest. Undoubtedly, tartaric acid is best for the taste of finished wine.
Naturally, most grape wines contains about 5 – 6 g/L acidity in tartaric acid equivalents (TAE). It is important to know that wine acidity is not expressed as TAE in all countries. In France, wine acidity is expressed in sulfuric acid equivalents (SAE). In order to convert SAE to tartaric acid equivalents, SAE should be multiplied by 1.53. So, a wine with an acidity of 3.9 g/L in France actually has an acidity of 6.0 g/L in TAE.
In my experience, the minimum TAE of wine should be 5 g/L and definitely no lower than 4 g/L. If the acidity of wine is too low, the risk of defects in the wine increases significantly.
You will hardly ever see the need to decrease acidity during winemaking. If needed however, you can reduce wine acidity by adding a calculated amount of Osorno’s Acidity Neutralizer (potassium carbonate). You can also add this neutralizer into finished wine. It is important to know however, potassium carbonate tends to absorb moisture, and is therefore not easily handled.
Example
Keeping in line with the rest of this book, our example calculation will be based on a target value of 10 L of finished wine.
Let us assume that we want to produce kiwi wine, starting with 2.5 kg kiwi. Kiwis typically contain 500 mg malic acid and 990 mg citric acid per 100 g. (These fruit acids are the main acids in kiwis so we can ignore other acids.)
Answer
This means that we have 5 g of malic acid and 9.9 g of citric acid per kg of kiwi, leading us to 12.5 g of malic acid and 25.5 g of citric acid in the 2.5 kg of kiwi that we intend to use.
Using Table 1 above, we can calculate that 12.5 g of malic acid is the equivalent of 11.1 g of tartaric acid, and that 25.5 g of citric acid is equivalent to 49.0 g of tartaric acid, resulting in a total acidity of 60.1 g (as TAE) for the 10 L batch. This is perfectly within the range of an alcohol-rich wine with residual sweetness (in other words, a dessert wine). If we allow this wine to age properly, we have to take into account that it will lose acidity through the loss of malic acid (owing to malolactic fermentation). In the end, the aged wine will still be above the recommended threshold of 50 g TAE.
Exercise
Calculate if 4 kg kiwi were used for a 10 L batch instead of 2.5 kg.
Answer
The acidity will increase well above a comfortable value, demonstrating a need for dilution with water. As this exercise shows, a calculation of the correct fruit to water ratio must always be the first step before calculating the amount of sugar that has to be added.
Wine Acidity and Colour
Obviously, uncoloured fruit (e.g: white grapes) does not give a coloured wine. It may surprise you to know that juice from red grapes is coloured almost like juice from white grapes. In grapes, the colour of wine mainly comes from the skin of the fruit, and can be released only by maceration.
Almost all natural fruit colours come from anthocyanins. Anthocyanins are the pigments that give red, purple, blue, and black colours to plants and fruits. Anthocyanins exist not just to enhance nature’s beauty, but also to protect nature from the sun’s UV rays.
Anthocyanins are most stable on the pH scale between 3 and 4, which nicely matches the conditions for winemaking. The best colour of wine can be attained when there is sufficient acidity during the maceration period.
It should be pointed out that the colour of all anthocyanins depends strongly on their pH — which makes them suitable as pH indicators. The colour of anthocyanins changes from red to purple, then blue, then black as pH goes up from 1 to 13. (This progression also explains why you cannot find blue roses; the pH of rose petals is within an acidic range). Needless to say, blueberry skin is slightly alkaline, or otherwise they would be “redberries”. From the information provided above, it should be clear why blueberry wine is dark red, and not blue.
It should be mentioned that anthocyanins are largely not bio-available for the human body. 99% of anthocyanins are not resorbed in the human intestines, but instead, they stay with their anti-oxidant properties in the intestines.
Despite many studies about the health effects of eating berries, it seems only in recent years that the bio-availability of anthocyanins has become a subject of research ( Iva Fernandes, Frederico Nave, Rui Gonçalves, Victor de Freitas, Nuno Mateus; Food Chem. 135,2 812 (2012). ).
Buying Fruit Acids
The price of fruit acids can vary widely. Some fruit acids are produced industrially (e.g: citric acid), while other fruit acids can only be found in nature, (e.g: tartaric acid). Osorno offers certain fruit acids for winemaking. To see collection, please visit our Chemicals Section.
If you would like to purchase fruit acids or receive advice about ingredients for winemaking, please visit the Osorno Store.
